Redox and Eh From electrochemistry: G R = -nF Eh – E° = - G R ° / nF – For e - on left side of half-reaction; – If e - on right side: E° = + G R. - ppt download

In vitro simulation of oscillatory redox conditions in intertidal sediments: N, Mn, Fe, and P coupling - ScienceDirect

Redox and Eh From electrochemistry: G R = -nF Eh – E° = - G R ° / nF – For e - on left side of half-reaction; – If e - on right side: E° = + G R. - ppt download

A new Amazon gift card scam is landing in inboxes - and it's really not very Christmassy Amazon - Wilson's Media

PDF) Impacts of Petroleum Activities for the Achuar People of the Peruvian Amazon: Summary of Existing Evidence and Research Gaps

Redox and Eh From electrochemistry: G R = -nF Eh – E° = - G R ° / nF – For e - on left side of half-reaction; – If e - on right side: E° = + G R. - ppt download

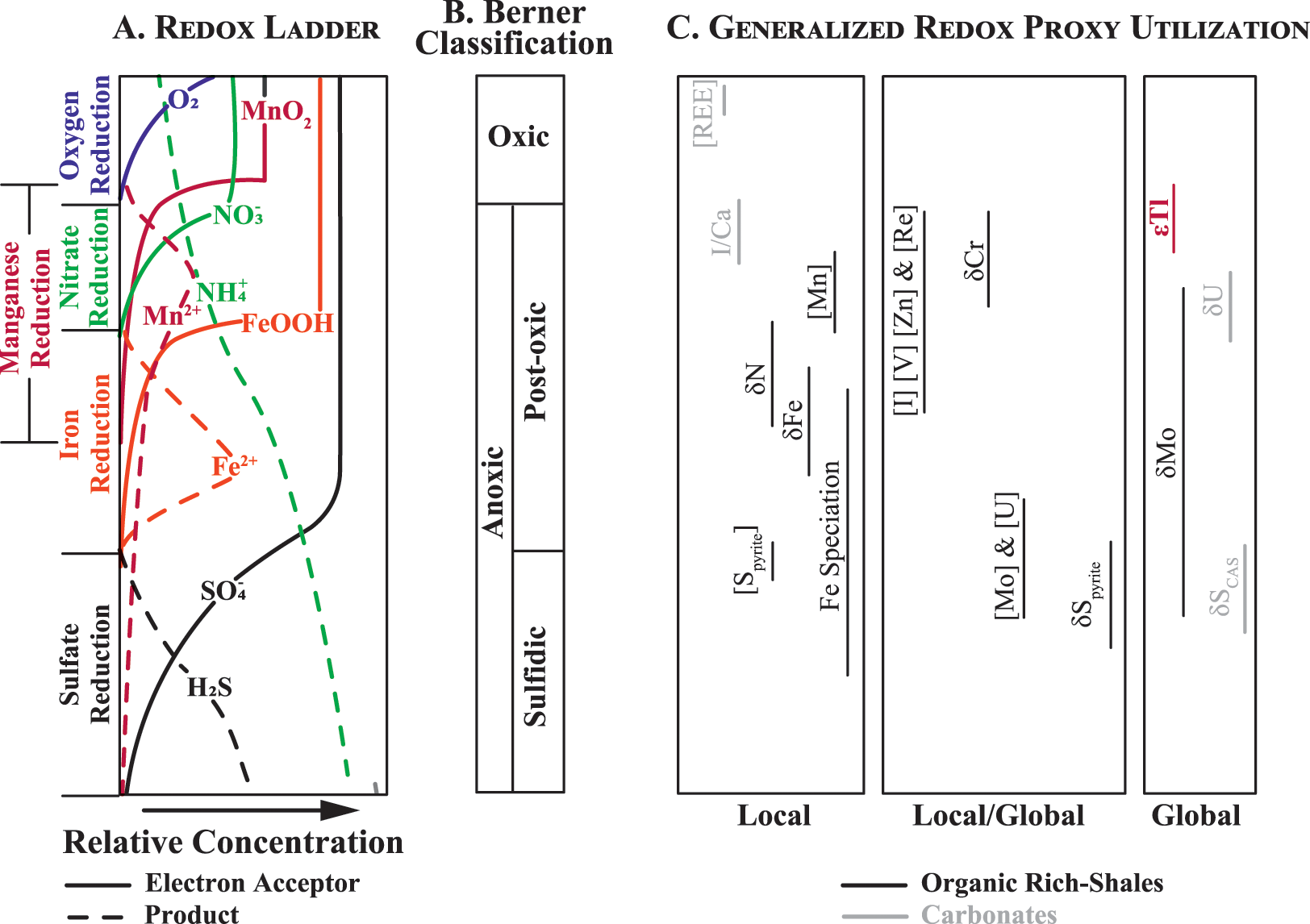
Redox ladder showing some examples of environmentally relevant redox... | Download Scientific Diagram
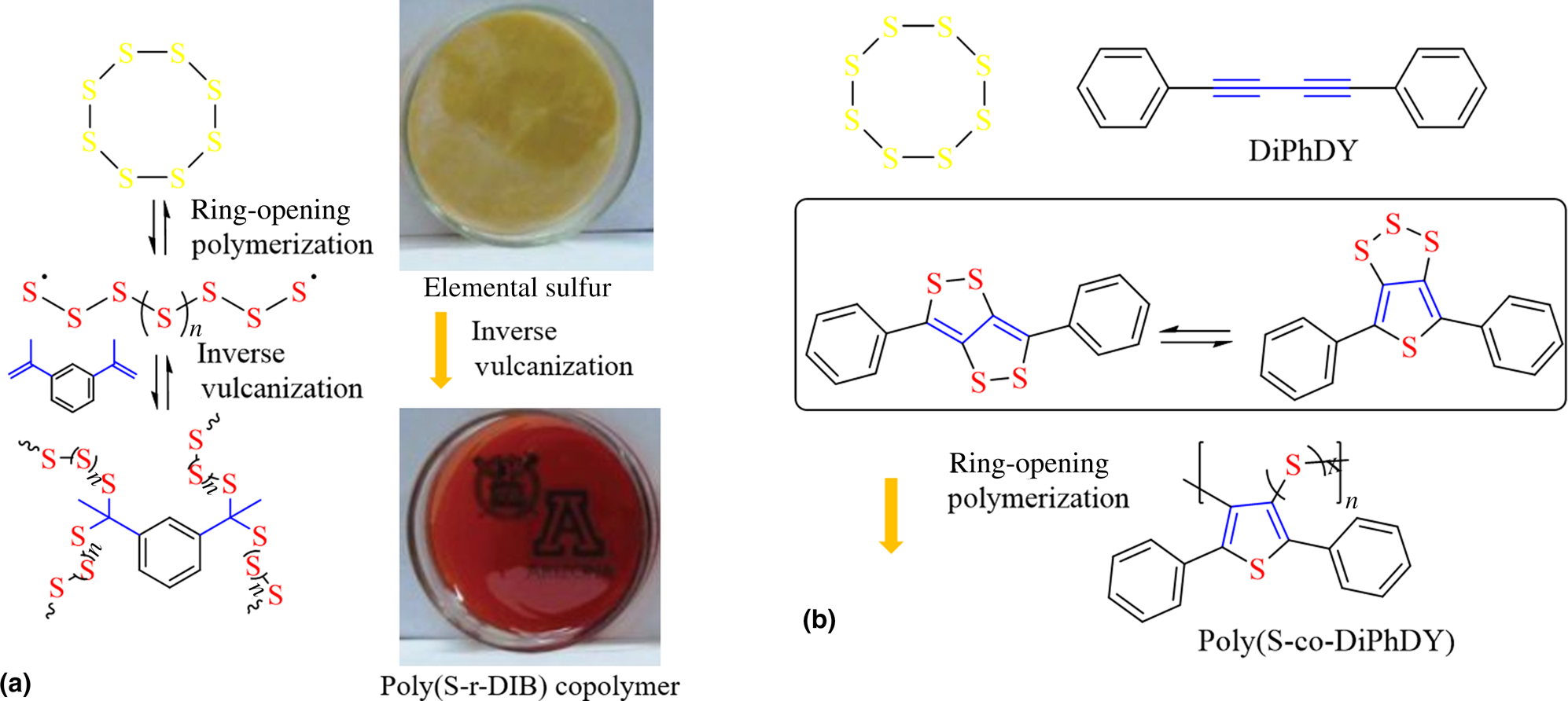
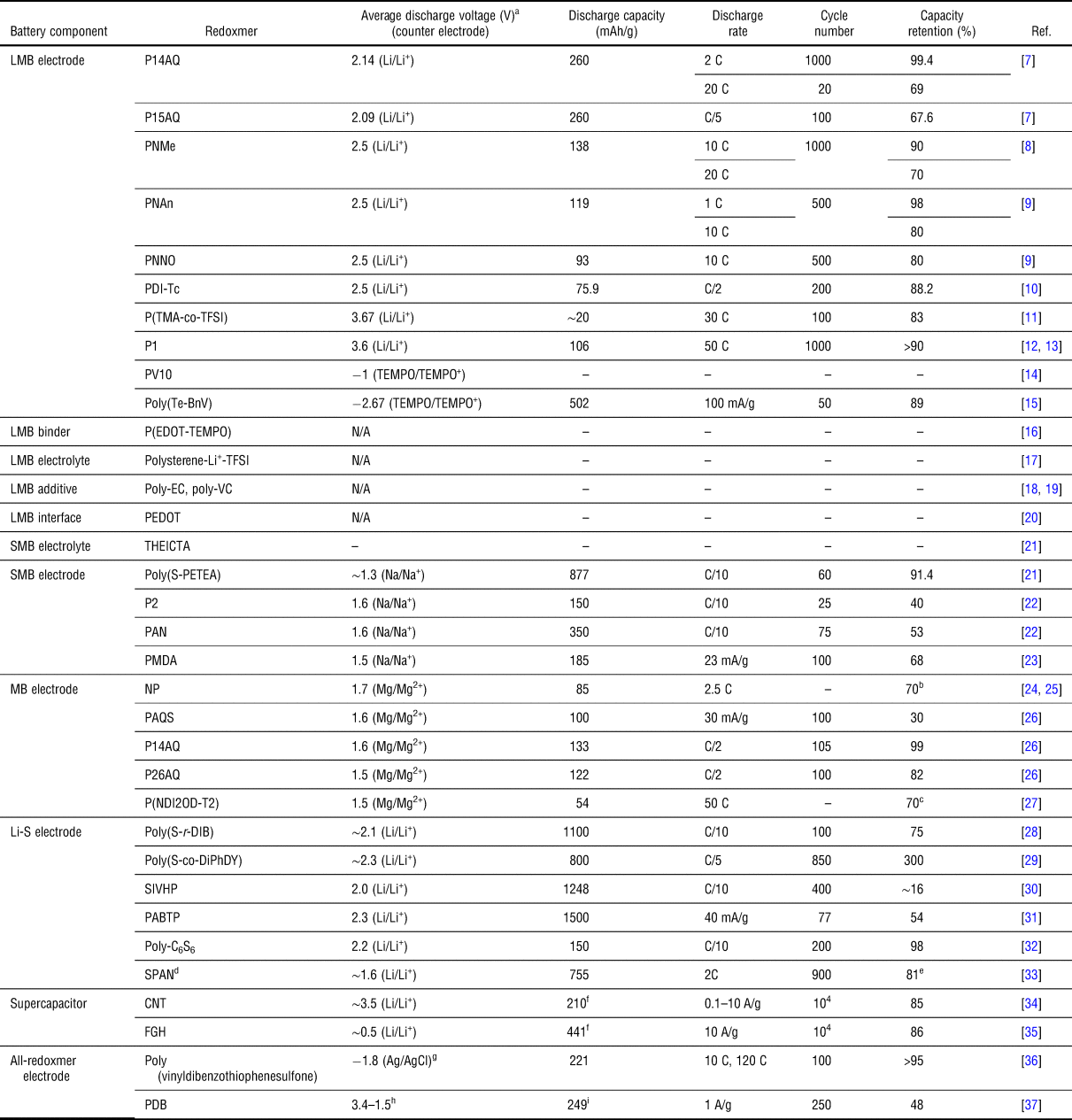
Redox-active polymers (redoxmers) for electrochemical energy storage | MRS Communications | Cambridge Core
Redox-active polymers (redoxmers) for electrochemical energy storage | MRS Communications | Cambridge Core

Dr. Karen Vaughan on Twitter: "The redox ladder is a concept that explains oxidation-reduction (or redox) couples along a stair-case (or ladder) with the most energetically favorable reaction at the top and

Redox and Eh From electrochemistry: G R = -nF Eh – E° = - G R ° / nF – For e - on left side of half-reaction; – If e - on right side: E° = + G R. - ppt download

Redox and Eh From electrochemistry: G R = -nF Eh – E° = - G R ° / nF – For e - on left side of half-reaction; – If e - on right side: E° = + G R. - ppt download