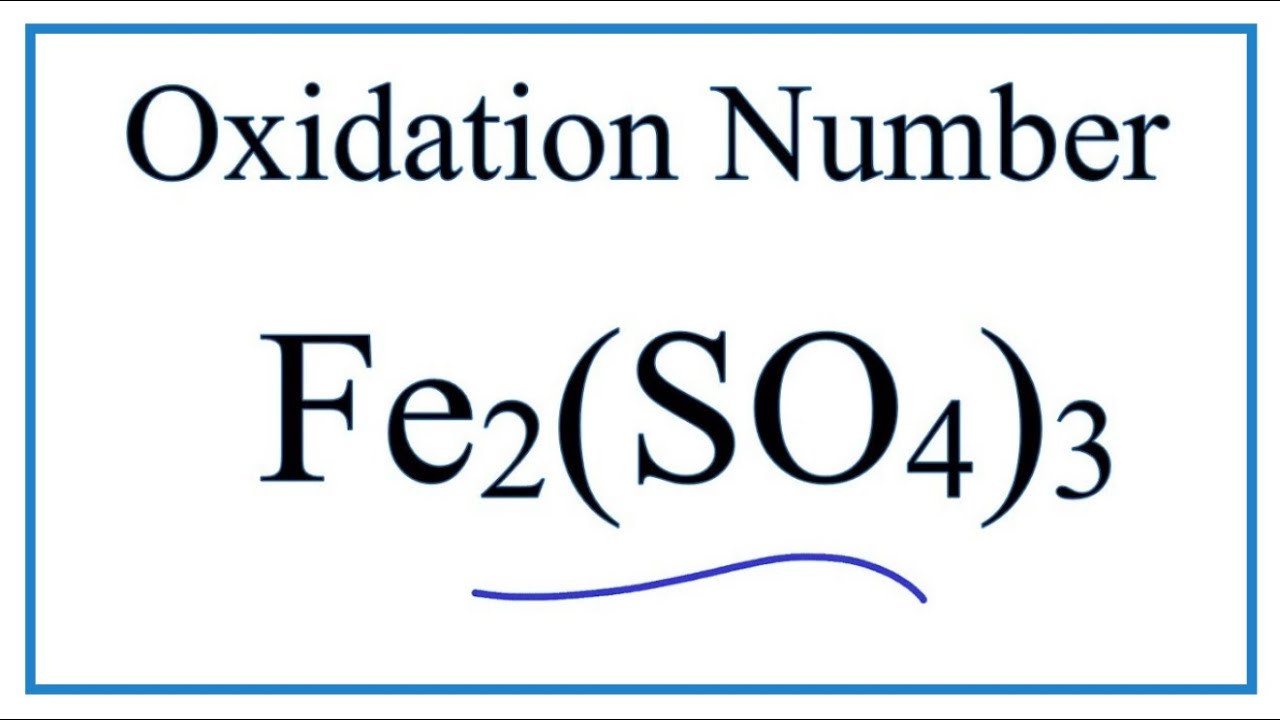
Question Video: Identifying Iron Oxide Produced from the Reaction of Unknown Salt with an Alkali Solution | Nagwa
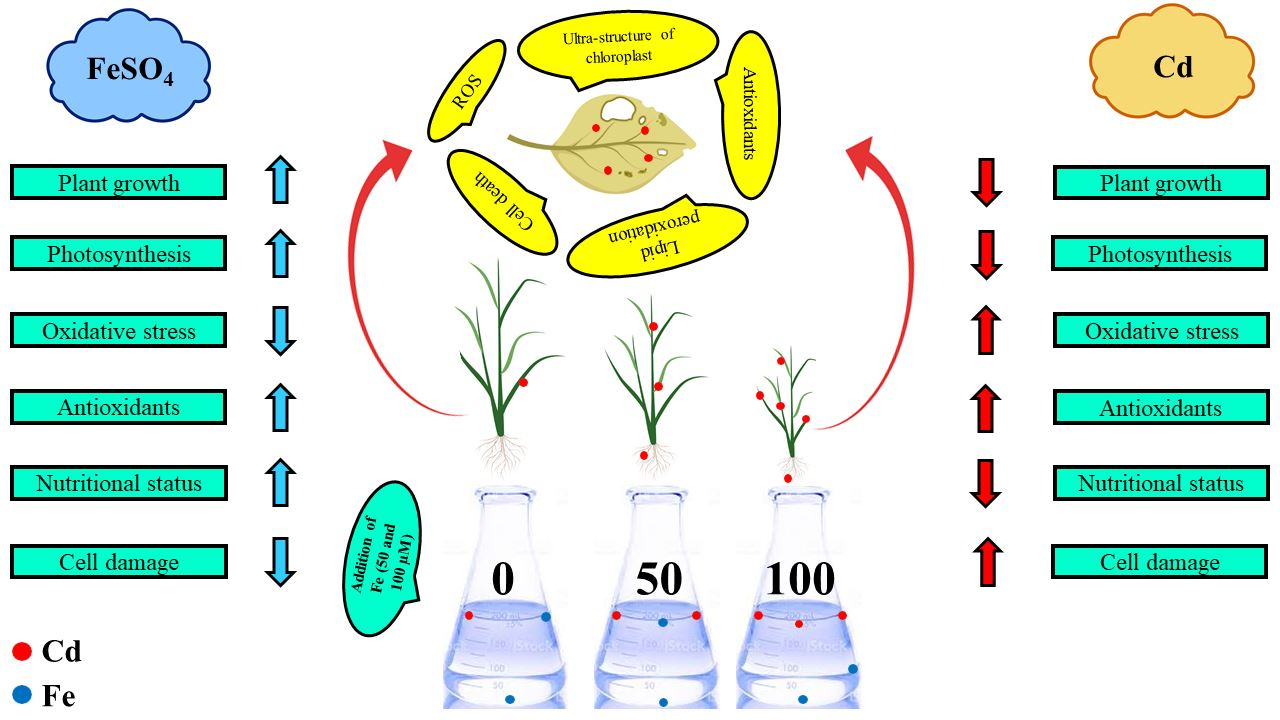
Biomolecules | Free Full-Text | Role of Ferrous Sulfate (FeSO4) in Resistance to Cadmium Stress in Two Rice (Oryza sativa L.) Genotypes

What is the colour of FeSO4 . 7H2O crystals ? How does this colour change upon heating ? Give balanced chemical equation for the changes.
In order to oxidise a mixture of one mole of each of FeC2O4, Fe2(C2O4)3, FeSO4 and Fe2(SO4)3 - Sarthaks eConnect | Largest Online Education Community

Iron(III) Sulfate as Terminal Oxidant in the Synthesis of Methyl Ketones via Wacker Oxidation | The Journal of Organic Chemistry
One mole of KMnO4 is used for complete oxidation of FeSO4, FeC2O4 and H2C2O4 in acidic medium respectively and separately. Pick up the correct statement : (1) 5 mole FeSO4 can be
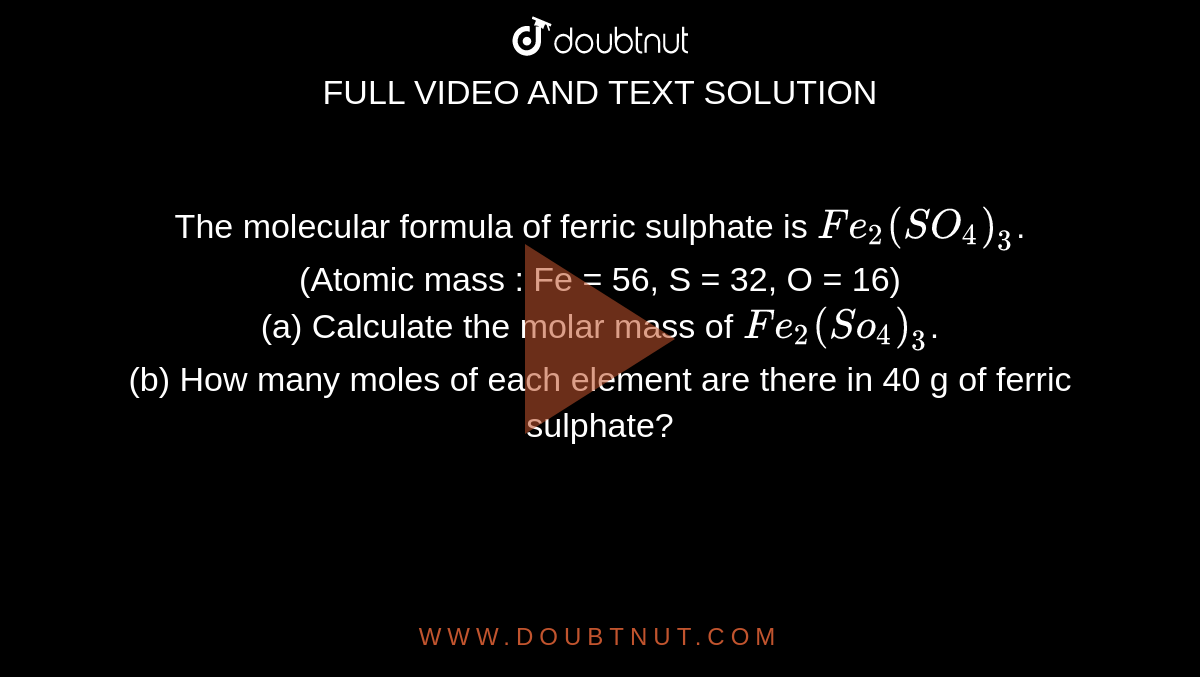
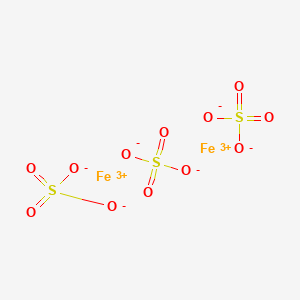
The molecular formula of ferric sulphate is Fe2(SO4)3. (Atomic mass : Fe = 56, S = 32, O = 16) (a) Calculate the molar mass of Fe2(So4)3. (b) How many moles of

In order to oxidise a mixture one mole of each of FeC2O4, Fe2(C2O4)3, FeSO4 and Fe2(SO4)3 in acidic medium, the number of moles of KMnO4 required is: